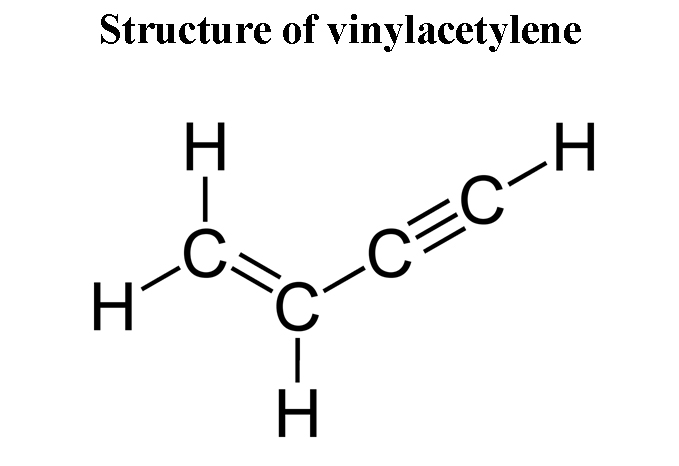
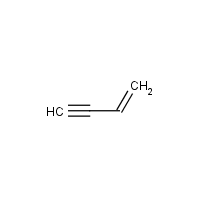
The IUPAC name of vinyl acetylene is: | 11 | CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUND... - YouTube

Catalysts | Free Full-Text | Selective Hydrogenation of Concentrated Vinyl Acetylene Mixed C4 by Modified Pd Catalysts: Effect of Cu
VINYL ACETYLENE 689-97-4, China VINYL ACETYLENE 689-97-4 Manufacturers, China VINYL ACETYLENE 689-97-4 Suppliers - intergas
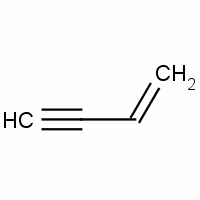
1. IUPAC name of vinyl acetylene is (a) but 1 ene 3 yne (b) but 1 yne 3 ene (c) both are correct (d) None of the above

Selective hydrogenation of mixed C4 containing high vinyl acetylene by Mn-Pd, Ni-Pd and Ag-Pd on Al2O3 catalysts - ScienceDirect
![The IUPAC name of vinyl acetylene is:A) \\[Pent-1-en-4-yne\\] B) \\[Pent-4-yne-1-en\\] C) \\[but-1-en-3-yne\\] D) \\[but-1-yn-3-ene\\] The IUPAC name of vinyl acetylene is:A) \\[Pent-1-en-4-yne\\] B) \\[Pent-4-yne-1-en\\] C) \\[but-1-en-3-yne\\] D) \\[but-1-yn-3-ene\\]](https://www.vedantu.com/question-sets/28b6190a-bfeb-450a-8b35-1562475ebb908961788802603575039.png)
The IUPAC name of vinyl acetylene is:A) \\[Pent-1-en-4-yne\\] B) \\[Pent-4-yne-1-en\\] C) \\[but-1-en-3-yne\\] D) \\[but-1-yn-3-ene\\]

CoC bond in between (C2-C3) vinyl acetylene is formed by ......... overlapping P ucation Exercise 4 (a) sp-sp (b) sp'-sp2 one is formed by ........... overlapping, 2. P. is a stable form